Background: Cutaneous T-Cell Lymphomas (CTCL) are rare malignancies that comprise <10% of Non-Hodgkin Lymphoma. Limited treatment options exist for these patients, especially after progression following front-line therapy. Tolinapant is an oral non-peptidomimetic antagonist of the inhibitor of apoptosis proteins (IAPs), cIAP1/2 and XIAP. It plays a role in regulated tumor cell death and immunomodulation (Ferrari et al., Blood Adv. 2021) and has been evaluated in the clinic (ASTX660-01 trial, NCT02503423). Previously, we reported an overall response rate of 28% in CTCL patients treated with tolinapant, with a median duration of response of 8.8 months (Michot et al., EHA 2022). During this trial, in which the patients were given tolinapant on Weeks 1 and 3 in 4-week cycles, clinical investigators have described multiple instances of tumor flare and pseudoprogression, consistent with the described immunomodulatory effects associated with tolinapant treatment.
Here, we present one such case, a 58-year-old male, who was diagnosed with mycosis fungoides in 2010, which had previously transformed despite multiple therapies. This subject was selected for a more detailed study due to their clinical course with multiple blood samples and biopsies collected, permitting longitudinal histological and molecular characterization. At enrollment in March 2021, the subject presented with red, scaly plaques and cutaneous tumors scattered on the extremities and trunk. At one-month follow up, the subject had an excellent initial symptomatic response with the visual resolution of several tumors. Moreover, the itch reported as “moderate” at baseline had improved with tolinapant treatment. However, during continued therapy, the remaining plaques and tumors progressively swelled, appearing pink and indurated on dosing, indicative of flare. During off-dosing weeks, these signs of flare were reduced. The subject also reported increased itch during treatment that partly resolved during off-dosing weeks. Considering the disappearance of some tumors and the improved appearance during off-dosing weeks, this was pseudoprogression.
Methods: Cell surface phenotype of the lymphoma was determined by flow cytometry (Standard Leukemia/Lymphoma and T-Cell Therapy Panel, NeoGenomics Laboratories) on blood samples collected at screening and during Cycles 1, 3 and 5. To characterize the clinical observations, skin biopsies including tumor tissues were collected at baseline and during Cycles 1, 2, 6, 7 and 10. We used multiplex immunofluorescence analysis (COMET™, Lunaphore Technologies SA), gene expression profiling (PanCancer IO 360™ and Pathways panels, NanoString Technologies, Inc.), genomic profiling (FoundationOne™ Heme, Foundation Medicine, Inc.) and cytokine/chemokine measurements (Human ExplorerMAP™ v. 1.0, xMAP® Technology, Luminex Corporation).
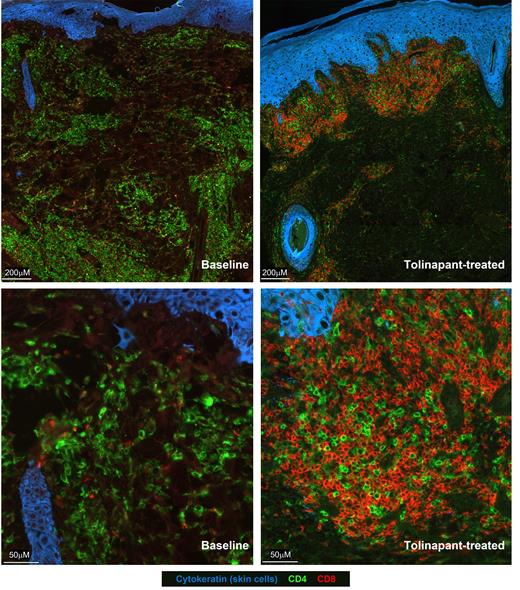
Results: Blood samples collected at screening and post-treatment confirmed the presence of lymphoma cells that were CD3 +, CD4 +, CD7 -, CD8 - and CD26 -. Multiplex immunofluorescence analysis of the biopsies was able to detect lymphoma cells as CD4 +, CD7 - and CD8 -. Sparsely distributed CD8 + cells were observed at baseline (screening sample). In the biopsy taken on Cycle 2 Day 1, the level of CD8 + cells was markedly increased. Representative images are shown below (left: baseline; right: post-treatment). Analysis of genes associated with CD8 + T cells, cytotoxic cells, B cells and NK cells showed an increase in gene probe signals for CD8A, CD8B, GZMA, GZMH and PRF1. Post-treatment plasma samples showed an elevation in chemokines involved in leukocyte recruitment, such as, CXCL1, CXCL10 and CCL25. Similarly, consistent systemic changes in CXCL10, the chemokine directing recruitment of CXCR3 + cytotoxic T cells, were also detected in tolinapant-treated peripheral TCL patients in the same trial.
Conclusions: Our data offer a cellular and molecular insight into a case of clinical pseudoprogression observed during tolinapant treatment. Understanding pseudoprogression is important for clinical investigators and patients to ensure treatment with tolinapant, or other IAP antagonists, are not discontinued prematurely.
Disclosures
Jueliger:Astex Pharmaceuticals: Current Employment. Davis:Astex Pharmaceuticals: Current Employment. Potapov:Astex Pharmaceuticals: Current Employment. Ward:Astex Pharmaceuticals: Current Employment. Smyth:Astex Pharmaceuticals: Current Employment. Taylor:Astex Pharmaceuticals, Inc.: Current Employment. Keer:Astex Pharmaceuticals, Inc.: Current Employment. Sims:Astex Pharmaceuticals: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal